comeSource | Yaozhi. com(Reprinted by Cybertron)
Write | three
Up to the seventh batch, Hengrui Pharma has won the bid for 20 varieties. During this period, however, the market value continued to be under pressure. Although there are many reasons for losing the bid, it is not that winning the bid can unilaterally bring different profits to the enterprise.
The answer is obvious.
Centralized purchasing is a hurdle that Hengrui can’t cross, and the same is true for the entire pharmaceutical industry. Nowadays, Hengrui innovative drugs are developing rapidly, and a large amount of R&D investment is made every year. Under the guidance of innovative drugs, Hengrui’s short-term pain at present may be just preparing for the bright future.
But is this really the case?
Looking back on the situation in the national medical insurance talks last year, the price of Karelizumab of Hengrui decreased by 85% and that of Esketamine decreased by 68%, but it failed to achieve the expected effect of "exchanging price for quantity". In the end, due to various factors, the sales volume of the product fell short of expectations, and the overall performance declined seriously in 2021. Even in the first quarter of this year, there was no sign of stopping the decline, and 20.93% of the performance decreased year-on-year, which was "Du Jie".
From this point of view, in today’s pharmaceutical market, it is not just generic drugs that are not easy to do, and the situation of innovative drugs is also not optimistic.
Last month, the National Medical Insurance Bureau officially announced the Work Plan for the Adjustment of the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List in 2022 and related documents. Hengrui has not recovered from the last shock, but the fifth round of the adjustment of the national medical insurance drug list will begin again.
This time, for Hengrui, is it a turning point, or is it worse?
01
Hengrui 3 new drugs, medical insurance is the only way out?
According to relevant reports, from July 1 last year to June 30 this year, there were about 31 innovative drugs (excluding newly approved indications for listed drugs) approved by domestic enterprises for the first time, including monoclonal antibodies, double antibodies, CAR-T and other drug types, covering many diseases such as cancer and immunity.
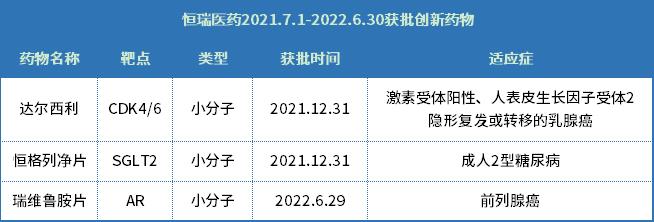
Among them, Hengrui has three new drugs that try to advance into the medical insurance catalogue this year, namely, Darceli, Hengglinide and Riverumide.
Heng ge lie Jing
First of all, as two new drugs approved by Hengrui on the same day, both Hengglinide and Darcyril are the first domestic innovative drugs in their respective targets.
The former, Henggliclazide, is also the first SGLT-2 1 new drug independently developed in China, which marks a major breakthrough in the field of treatment in China, and what is more important, of course, is to break the monopoly of foreign companies in the original research of SGLT-2 inhibitors for many years.
According to relevant data, up to now, five SGLT-2 inhibitors have been approved for listing in the domestic hypoglycemic drug market (including compound preparations). Because the approval time of Hengglinide is shorter than that of its predecessors, this has become its most important fatal factor. At present, the market is not fully opened, so it may be even more urgent to superimpose the medical insurance volume in this national medical insurance talk.
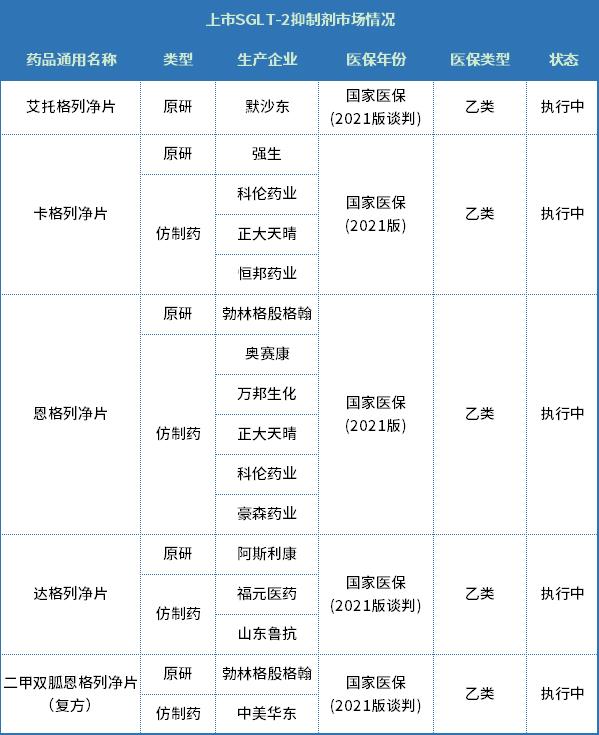
In addition, except for the generic drugs of Agglinide tablets in the above table, the other four SGLT-2 inhibitor competing products of Hengrui have been approved to be listed and regarded as over-evaluated, among which both Kagglinide and Engelglinide were included in the fourth batch of centralized purchase. Due to the late listing, the impact of overlapping centralized purchase did not fully appear, and the sales growth rate of the two products in China public medical institutions in 2021 still exceeded 500% and 200%.
Finally, although there are no other SGLT-2 inhibitor Class 1 new drugs approved in China except Hengrui Henggliclazide, the related products of Dongyang Guangyao, Xuanzhu Medicine and other enterprises have also entered Phase III clinical trials, and it is expected that more enterprises will join the competition pattern in the later period. It can be said that this year’s national health insurance talk has become the only hope of Hengrui, a new drug that has not been listed for one year.
Darsili
The situation of another new drug, Dalxil, is also not weaker than that of Hengglinide. According to relevant data, before the approval of Darcyril Tablets, there were four CDK4/6 inhibitors on the global market. Except Trilaciclib, which was approved for bone marrow suppression, the other three inhibitors (Piperacil from Pfizer, Rebocili from Novartis and Agbesi from Lilly) were all used to treat HR+/HER2- breast cancer.
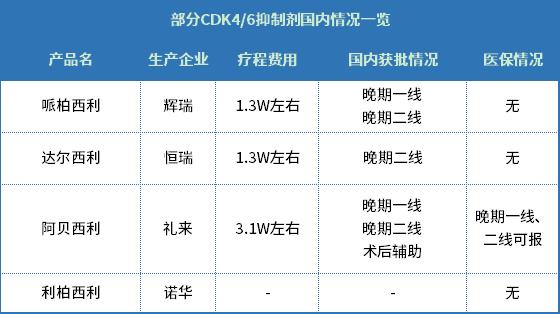
In last year’s national talks on medical insurance, Lilly negotiated successfully before Pfizer, and Abeceli became the first CDK4/6 inhibitor to enter the medical insurance catalogue, which was officially implemented on January 1, 2022. This year, in addition to Abeceli’s soon-approved auxiliary indications, it is highly probable that he will strive to enter the medical insurance list, and the remaining Pipeceli and Rebosi are expected to make many moves in this national talk. At the same time, CDK4/6 is the star target, and the innovative drug market in China has naturally become the priority layout direction of many enterprises. Even some enterprises have prepared generic drugs in order to avoid the overcrowded innovative drug track. On December 18, 2020, Qilu Pharmaceutical’s piperacillin generic drug (the first imitation) was approved by the Food and Drug Administration, waiting for the piperacillin patent to expire in 2023 and enter the market at any time. After Qilu, more than 20 companies also took a fancy to piperacillin generic drugs. Only five companies, including Kelun Pharmaceutical, Osekan, Haosen, Xiansheng and Shanxiang Pharmaceutical, are in the stage of listing application. Even the same innovative drug track also has more than ten products in the clinical stage, such as Zhengda Tianqing, Beierda Pharmaceutical and Jinzhou Aohong. Although the market prospect is attractive, its involution degree is even worse than that of PD-1/L1, and Darceli of Hengrui, even if the national health insurance talk is successful, has little advantage in price and time to market regardless of efficacy. Not surprisingly, the drug of Hengrui will appear at an "amazing" price reduction in this national talk.
Rivirutamide
As for the last Rivirutamide, as a new drug that entered the division time on June 29th, it is mainly used for metastatic hormone-sensitive prostate cancer with high tumor load, and it is also the first new androgen receptor (AR) inhibitor independently developed by China. According to the publicity content of Hengrui, compared with the standard treatment, the overall survival OS of Rivirutamide has been significantly prolonged, and the risk of death and disease progression has been significantly reduced by 42% and 54% respectively. However, it is still unknown whether it can provide a brand-new excellent treatment scheme at present, and it will take some time to be verified by the market.
02
The "involution" of PD-1 situation is still the theme.
Of course, in addition to the above three new drugs, Hengrui’s national talk about PD-1 this year is also one of the most concerned contents and one of the biggest attractions.
First of all, Karelizumab is the only PD-1 monoclonal antibody in the current catalogue that is about to expire. On the premise of the failure of negotiations on nasopharyngeal carcinoma last year, plus the new indications for first-line esophageal cancer and first-line squamous NSCLC this year, the number of items to be discussed increased to four this year.
At the same time, because esophageal cancer and NSCLC are the major diseases with the highest morbidity and mortality in China, it is expected that this year it is very likely to form a "packaged" admission situation-four indications will enter medical insurance at the same time.
Of course, the opposite must be a huge price reduction (refer to the previous PD-1 price reduction and renewal, perhaps even more).
More crucially, since the second half of last year, domestic PD-(L)1 monoclonal antibodies have been approved one after another, and three domestic PD-1 and two domestic PD-L1 may also join the battle in this national medical insurance talk.
This year’s PD-1 national health insurance talk must be another bloody storm.
03
summary
On the whole, perhaps innovation is indeed the way out for Hengrui, but this road is not as smooth as expected.
At least for the time being, Hengrui, who has temporarily passed the centralized mining safely, may face the real problem of "future" in the next step. The innovative drug market, which looks like scenery, is also full of crises, and involution will become the most important rhythm in the next few years or even decades.
In recent years, under the influence of a number of policy adjustments, leading enterprises such as Hengrui are far less able to accurately control and grasp the main trend of the market, and the advantages they can show when competing with biotech enterprises are not as good as before.
The only way to finally change the present situation is time.
关于作者